INFORMATION GUIDE
STEEL
Introduction
Protecting
a steel vessel from corrosion, requires absolute dedication to correct preparation
procedures if the selected system is to have any chance at all of succeeding.
To get good adhesion the surface must be cleaned to white metal and then prime
coated immediately, The most successful way to achieve this is sandblasting,
which not only cleans large areas quickly, but leaves an uncontaminated, and
slightly roughened profile for the primer to bond to.
The sandblasting
procedure is a professional job and should only be carried out by qualified
applicators. These people are experienced in the various degrees (or standards)
of clean metal states, and can produce the level specified by the paint manufacturer.
Usually the minimum requirement is S.A. 2 112 near white metal, or S.A.3 white
metal, Alternatively, A.S. 1627.4 should be considered as the minimum level.
The white metal state is seen as a uniform white metal surface without patches
of grey. Once this state is achieved, it is essential that the primer coat
be applied immediately, to seal off the steel from atmospheric moisture (which
will start the corrosion process all over again). Co-ordination of the blasting
and painting will require careful planning and optimum weather conditions.
SELECTING
A PAINT SYSTEM -EXTERNAL SURFACES
This
can be confusing and needs some careful evaluation of:
a. The
areas to be coated,
b. The
type of use the surface will be put to,
c. What
degree of finish will be required,
d. What
maintenance cycles are contemplated and
e. What
geographical environment will be involved.
Single
Pack Paint systems
These
offer the simplicity of easy recoating and generally are more tolerant of
application temperatures. On the
other
hand, they are usually solvent sensitive and not as abrasive resistant. They
can only be used successfully as above
water
coatings because they are only water resistant, not waterproof, and will swell
up and blister under constant immersion.
2 -Pack Paint systems
They
offer higher film build protection on a coat for coat basis, but can give
inter-coat adhesion problems if incorrectly applied. Recoating also requires
more preparation to obtain a satisfactory bond, but offers considerably less
maintenance. To compound the problem of paint selection, each area on a steel
boat usually has a different performance requirement. This requires close
analysis of the coatings ability to provide value for money, in the longer
term. For example, a yacht may require considerable freeboard fairing with
epoxies to eliminate hollows in plate welding whereas a commercial steel trawler
will ignore variations in hull smoothness and paint the area in a low sheen
finish to mask the surface irregularities.
DECKS
As this
surface has the highest heat absorption value, the coatings should be capable
of distorting to a similar co-efficient of expansion to that of the steel.
If the paint fails to flex in a similar manner, it will crack and allow corrosion
to develop.
If the
paint is too soft, it will not withstand traffic. Therefore, it should be
hard enough to withstand abrasion but still be sufficiently flexible to cope
with the steel distortion.
TOPSIDES .
Although
the distortion factor will be less than the deck area, the colour, quantity
of fairing filler to be used, and abrasion
resistance
will also dictate the selected finish.
BELOW
WATERLINE
The use
of an anti-fouling paint over the protective primers also provides another
dimension due to the fact that these metals will cause an electrical current
to flow through the Electrolyte (seawater) causing the dissolution
of one of the metals (unless adequately insulated). To overcome this problem
cathodic protection principles are employed to maintain the integrity of the
hull, and this requires professional advice to obtain the best results.
The basic
principles of corrosion and cathodic protection are listed below for your
consideration. CATHODIC PROTECTION
When
two different metals are in contact and immersed in an electrolyte (i.e. an
electrically conducting solution such as seawater), an electric current flows
from one metal to the other through the electrolyte. Associated with this
flow of current is the dissolution of one of the metals. This phenomenon we
recognise as corrosion.
The composition
of the electrolyte plays only a minor part and consequently it is possible
to arrange metals in a table such that for any pair, the one higher in the
table will corrode in preference to the other. Because this is an electrical
phenomenon, a certain voltage or potential difference exits between any pair
of metals. If one metal is taken as a reference standard for the scale, it
is then possible to ascribe a specific voltage to each metal in the table.
In practice, hydrogen is taken as zero and after standardising a number of
variables, the 'electro-chemical series of metals' has been evolved. The following
have been extracted from that table.
- Magnesium -2.38 volts
- 2. Aluminium -1.66 volts
- 3. Zinc- 0.76 volts
- 4. Iron -0.44 volts
- 5. Lead -0.12 volts
- 6. Hydrogen 0
7 Copper + 0.34 volts
8.
Silver + 0.80 volts c,..-t
- Gold + 1.36 volts
Taking
iron and copper as an example, the iron being higher in the table will corrode
at the expense of the copper. The potential of the iron relative to the copper
is: -O.44V -(+0.34)v = -0.78V or minus 780 millivolts. This latter
figure
will v~ry SO~ewhat in pra~tice depending. on the ~atu~e of the electrolyte
and its temperature. 1
So far,
consideration has been given only to pairs of quite different metals. It happens
that there are t frequently minor potential differences over the surface of
a single metal, steel for example, and hence it will corrode in the absence
of a second metal. A great deal of work has been carried out on steel and
millscale and the potential of the former to be approximately -450mV, a quite
substantial value.
When
corrosion occurs, the flow of electric current is accompanied by chemical
reactions. The phenomenon is
1therefore
more than an electrical one, it is electro-chemical and some of the terms
that are used have been borrowed from that science. The words anode and cathode
are applied to the two metals of a cell. The metal which corrodes or has the
lower potential or is nearer the top of the above table is the anode, the
other is the cathode.
The electro-chemical process of corrosion.
In the
simple case of steel sheet partially covered with millscale and immersed in
seawater, iron ions carrying two positive charges (Fe++) pass into solution
at the anodic areas and electrons (-) migrate within the steel from the anodic
to the cathodic areas thereby establishing an electric current.
EXTERIOR
Unpainted Surfaces
1. Remove
surface contamination (grease, oil etc.) with NORGLASS WAX & GREASE REMOVER,
and hose off salt deposits.
2. Abrasive
blast clean to AS1627.4 or S.A. Class 21/2 to 3 (near white or white metal).
Remove all sand and dust, and apply
the first
coat within 6 hours or as soon as possible. If the surface is contaminated
by rain, spray or mist, reblasting will be necessary.
INTERIOR Unpainted Surfaces
1. As
above where possible.
2. If
abrasive blast cleaning is not carried out, power sanding to a clean metal
state may be acceptable as an alternative.
Remove
all dust and apply the first coat within 6 hours or as soon as possible.
Previously
Painted Surfaces (in good condition)
1. Remove
grease and oil with NORGLASS WAX & GREASE REMOVER and wash
with fresh water to remove salt deposits. Allow to dry
2. Sand
to a smooth, flat surface and remove dust
Previously
Painted Surfaces (in poor condition)
1. Where
the adhesion of previous coats is poor, removal by blast cleaning is recommended.
If the bare steel has not been penetrated, then scraping, wire brushing, and
sanding, should be sufficient.
2. Sand
to a smooth flat surface and remove dust.
PRIMING
*HULL
EXTERIOR: (Above and Below waterline)
Apply
a minimum 2 coats of NORGLASS EPOXY TAR as directed on the Data sheet
and label instructions.
*HULL
EXTERIOR: (Alternative above waterline system. (From the top of the boot-topping
line).
Immediately
after sandblasting, apply 3 coats of NORGLASS NoRUST Primer as directed.
After the final coat allow 2 hours to cure and re-mask the waterline from
the top of the boot-topping. Below waterline areas require NORGLASS EPOXY
TAR.
*DECKS/SUPER-
STRUCTURE:
Same NoRUST recommendation as for above
waterline area.
*INTERIOR:
As per Decks/Superstructure, continue using NoRUST
as specified. UNDERCOATING *HULL EXTERIOR: Above and below the
waterline)
Apply
a minimum 2 coats of NORGLASS SHIPSHAPE PRIMER UNDERCOAT.
*DECKS/SUPER-
STRUCTURES:
As per
Hull exterior undercoating. Where practical the use of WEATHERFAST UNDERCOAT
can be used as an alternative to SHIPSHAPE.
*INTERIOR:
(Bilge area to waterline). Use only SHIPSHAPE
PRIMER-UNDERCOAT.
*INTERIOR:
(Above waterline)
Apply
2 coasts of NORGLASS WEATHERFAST UNDERCOAT.
FAIRING
*HULL
EXTERIOR: (Above waterline area)
Where
required NORGLASS NORFLEX EPOXY FILLER can be applied by trowel or
rubber squeegee. Mixing ratio 2:1 by volume. Note: Additional coats of NORGLASS
SHIPSHAPE will be required to cover the NORFLEX FILLER after fairing
and sanding has been completed.
*DECKS/SUPER-
STRUCTURE:
As above.
FINISHING
*HULL
EXTERIOR: (Below waterline and including boot-topping).
.Apply
2 coats of NORGLASS TOPFLIGHT ANTI-FOULING as directed on Data sheet
and label instructions.
*HULL
EXTERIOR: (Above waterline options)
(a) Apply
2 coats of WEATHERFAST GLOSS ENAMEL.
(b) Apply
2 coats of WEA THERFAST GLOSS ENAMEL white blended on a 50:50 ratio
with WEATHERFAST UNDERCOAT.
This
will produce a free chalking white semi- gloss finish that will mask plate
distortions. (c) Apply 2-3 coats of NORTHANE Gloss.
*DECKS:
Apply 2 coats of WEATHERFAST DECK PAINT. Note: Where smooth perimeters are
desired pre-coat these areas with WEATHERFAST ENAMEL first then mask out the
SLIP RESISTANT areas.
*SUPERSTRUCTURE: Apply 2 coats of WEATHERFAST MARINE ENAMEL. *INTERIOR: (Bilge area)
No further treatment required. *INTERIOR: (Above waterline).
Apply
2 coats of WEATHERFAST ENAMEL.
*ALLOY
MASTS & SPARS:
Refer
to the NORGLASS guide on Painting Aluminium for options.
*BRIGHTWORK:
Refer to the NORGLASS data sheets on Weatherfast Poly Clear or Marine Varnish
for options.
*ESTIMATING
MATERIAL REQUIREMENTS: All coverage rates expressed
are theoretical and do not al)ow for losses or spillage.
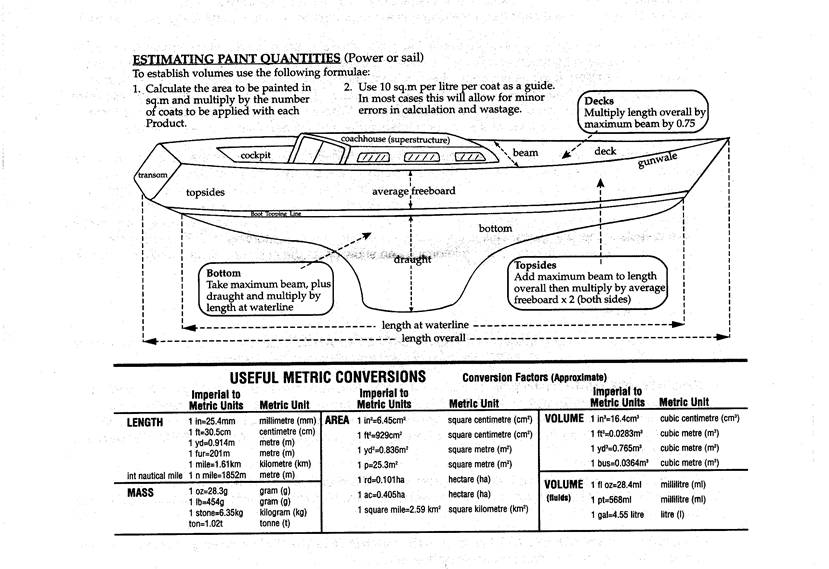
Where
a 2 pack product expresses a coverage rate this relates to the mixed product.
Do not include spraying thinners in these calculations and remember to multiply
areas by the recommended number to be applied.
SPECIFIC
INFORMATION
Norglass,
provides information data sheets on the following products for your assistance.
NoRUST
PRIMER* NORFLEX EPOXY FILLER* SHIPSHAPE PRIMER/UNDERCOAT* NORGLASS EPOXY
TAR*
WEATHERFAST ENAMEL * WEATHERFP.ST DECK PAINT* TOPFLIGHT ANTI-FOULING* SOFT
COPPER ANTI-FOULING* NORTHANE GLOSS.
NORGLASS
ADVISORY SERVICE
Ph: (02)
9708 2200 or Fax: (02)9796 3069. -Email: norglass@bigpond.net.au www.norglass.com.au

